Cabapan Cabazitaxel 60mg
Price 20000 INR/ Box
Cabapan Cabazitaxel 60mg Specification
- Dosage Form
- As Per Suggestion
- Origin
- India
- Feature
- Other
- Ingredients
- Other
- Application
- Other
- Storage Instructions
- Cool & Dry Place
- Shelf Life
- 2 Years
Cabapan Cabazitaxel 60mg Trade Information
- Minimum Order Quantity
- 1 Box
- FOB Port
- Delhi, Mumbai
- Payment Terms
- Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Paypal, Western Union, Telegraphic Transfer (T/T), Cash in Advance (CID), Cheque, Cash Advance (CA)
- Supply Ability
- 10000 Boxes Per Month
- Delivery Time
- 7 Days
- Packaging Details
- Per Box 1 vial
- Main Export Market(s)
- Australia, North America, Eastern Europe, Middle East, Western Europe, Africa, Central America, South America, Asia
- Main Domestic Market
- All India
About Cabapan Cabazitaxel 60mg
A drug called Cabapan Cabazitaxel 60mg is used to treat cancer. It contains Cabazitaxel, a taxane derivative that prevents the proliferation of cancer cells by interfering with the production of microtubules. The drug, which is normally given intravenously, is mainly used to treat metastatic castration-resistant prostate cancer that has progressed following chemotherapy based on docetaxel. Cabapan is intended to reduce tumour size and increase survival rates in people with advanced prostate cancer. However, it could result in negative side effects as fatigue, nausea, diarrhoea, and bone marrow suppression. According to the patient's health status, the dosage and treatment plan are chosen, and both should be delivered under a doctor's supervision.
Cabapan Cabazitaxel 60mg Features Include:
1. Cabazitaxel, a derivative of a taxane used in cancer treatment, is an active ingredient in cabagan.
2. The drug is offered in a 60mg dosage, ensuring accurate administration and dosing.
3. Delivery Through Intravenous Infusion: Cabapan is given via intravenous infusion, enabling effective distribution throughout the body to target cancer cells.
Potential Advantages:
1. Cancer Treatment: Cabapan Cabazitaxel 60 mg is mainly used to treat metastatic castration-resistant prostate cancer that has advanced following chemotherapy that contains docetaxel.
2. Cabazitaxel disrupts the production of microtubules, which prevents the growth and division of cancer cells.
3. Cabapan seeks to reduce tumour growth and increase survival rates in men with advanced prostate cancer.
4. Cabazitaxel is a type of targeted therapy that targets cancer cells while sparing healthy cells, minimising side effects.
5. Convenience: Effective delivery and dosage control are made possible by intravenous injection.
6. Combinational therapy: It can be used in addition to other forms of therapy to improve therapeutic results.
Always speak with an oncologist or other healthcare professional to learn more about the specific advantages and potential risks of Cabapan Cabazitaxel 60mg in your particular situation. Since each patient's response to therapy can differ, individualised medical guidance is crucial for providing the best possible cancer care.
Cabapan Cabazitaxel 60 mg is used for:
The primary indication for the usage of Cabapan Cabazitaxel 60mg is the management of metastatic castration-resistant prostate cancer (mCRPC), which has progressed following prior docetaxel-based chemotherapy. With the goal of reducing tumour growth and extending overall survival, it is a beneficial therapy choice for patients with advanced prostate cancer.
Cabapan Cabazitaxel 60mg Side Effects:
Cabapan Cabazitaxel 60mg may have a number of adverse effects, and each person may likely experience them to varying degrees. Typical negative consequences could be:
1. Low blood cell counts from bone marrow suppression can increase the risk of infection, anaemia, and bleeding issues.
2. Fatigue: A common adverse effect of treatment is feeling exhausted or weak.
3. GI Problems: It's possible to experience nausea, vomiting, and diarrhoea.
4. Peripheral neuropathy: Hands or feet may experience tingling or numbness.
5. Hair Loss: Alopecia, or temporary hair loss, is a potential adverse effect.
6. Anaemia: A decline in the number of red blood cells may result in anaemia.
7. Reactions to the intravenous infusion: Some people may have reactions during the infusion.
8. Changes in renal Function: For certain people, this may have an impact on renal function.
9. Rarely, serious allergic responses may manifest themselves.
10. Low white blood cell counts may increase the risk of infections, which could lead to an increase in infection rates.
Any troubling symptoms or side effects must be immediately discussed with your healthcare staff. They may offer you tailored advice and support during your cancer treatment and, if necessary, change your treatment plan to minimise side effects and maximise the advantages of Cabapan Cabazitaxel 60mg.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
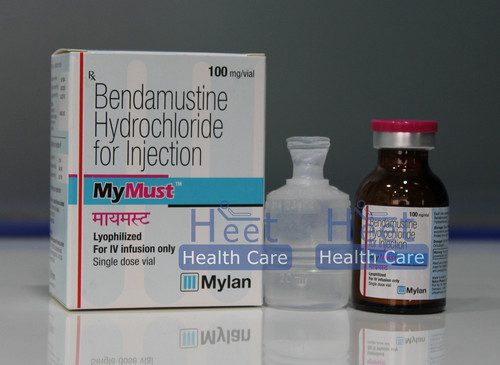
MyMust Bendamustine Hydrochloride 100mg
Price 3500 INR / Box
Minimum Order Quantity : 1 Box
Application : Other
Dosage Guidelines : As guided by physician
Physical Form : Liquid
Feature : Other
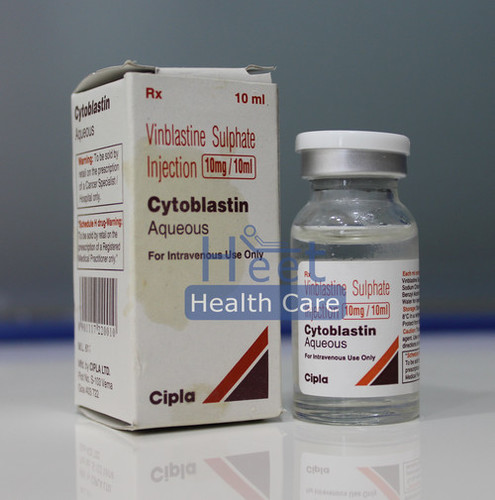
Cytoblastin Vinblastine Sulphate 10mg/10ml
Price 250 INR / Box
Minimum Order Quantity : 1 Box
Application : Other
Dosage Guidelines : As guided by physician
Physical Form : Liquid
Feature : Other
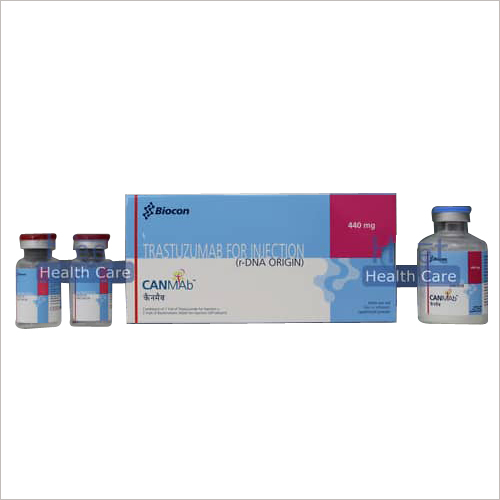
Canmab Trastuzumab 440mg Injection
Price 35000 INR / Box
Minimum Order Quantity : 1 Unit
Application : Other
Dosage Guidelines : As guided by physician
Physical Form : Liquid
Feature : Other
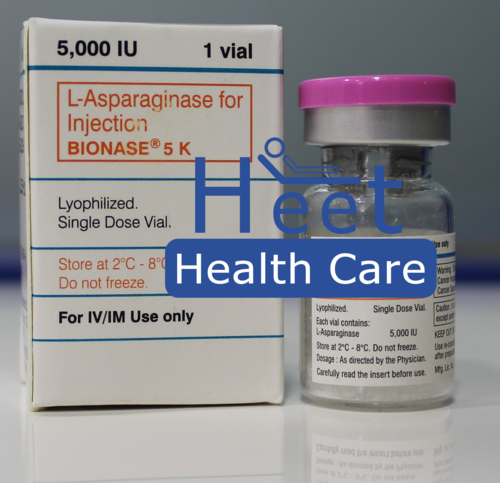
Bionase Asparaginase 5000IU
Price 1200 INR / Box
Minimum Order Quantity : 1 Box
Application : Other
Dosage Guidelines : As guided by physician
Physical Form : Liquid
Feature : Other
 |
HEET HEALTHCARE PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |

 Send Inquiry
Send Inquiry English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese Send Inquiry
Send Inquiry