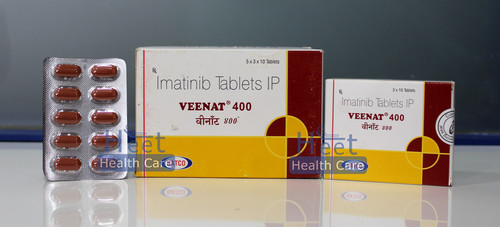
Veenat Capsules
Veenat Capsules Specification
- Origin
- India
- Dosage Form
- Capsule
- Enzyme Types
- Veenat Capsules
- Fermentation Smell
- Normal Smell
- Storage Instructions
- Dry & Cool Place
- Shelf Life
- 1 Years
Veenat Capsules Trade Information
- Minimum Order Quantity
- 1 Box
- Supply Ability
- 1000 Boxes Per Month
- Delivery Time
- 7 Days
- Main Export Market(s)
- Australia, South America, Eastern Europe, Middle East, Western Europe, Africa, Asia, Central America, North America
- Main Domestic Market
- All India
About Veenat Capsules
Imatinib, a tyrosine kinase inhibitor used to treat specific cancers, is the active component of Veenat capsules. It is typically recommended for gastrointestinal stromal tumours (GISTs) and chronic myeloid leukaemia (CML). Imatinib functions by preventing particular proteins from promoting the proliferation and development of cancer cells. Veenat promotes remission, slows the spread of cancer, and raises overall survival rates. The kind and stage of the cancer, as well as the patient's reaction to treatment, determine the dosage and length of the course of treatment. In order to control any side effects and guarantee the best treatment results, routine medical monitoring is essential.
Features and Advantages of Veenat (Imatinib) Capsules:
Features:
1. Tyrosine kinase inhibitor imatinib, the active component of Veenat capsules, is a tyrosine kinase inhibitor.
2. Veenat is generally given for the treatment of gastrointestinal stromal tumours (GISTs) and chronic myeloid leukaemia (CML).
3. Tyrosine Kinase Inhibition: Imatinib inhibits certain proteins known as tyrosine kinases that are essential for the growth and division of cancer cells.
4. Veenat provides tailored therapy that specifically targets cancer cells while sparing healthy cells.
5. Veenat is administered orally in the form of capsules, making it convenient for patients to give at home.
6. Personalised Treatment: Veenat enables a personalised treatment strategy depending on the particulars of the patient and the features of the cancer.
Benefits:
1. Veenat is a key component in the management of chronic myeloid leukaemia (CML), which improves patient outcomes and prolongs the duration of disease control.
2. Remission induction: Veenat aids in the process of remission, in which the quantity or visibility of cancer cells declines.
3. Prolonged Survival: Veenat has been demonstrated to increase patients' chances of long-term survival in cases of CML and GIST.
4. Slower Cancer Progression: Veenat reduces the growth and multiplication of cancer cells by blocking tyrosine kinases.
5. Better Tolerance: Compared to traditional chemotherapy, Veenat is often well-tolerated and has tolerable side effects.
6. Veenat capsules can frequently be administered as an outpatient procedure, eliminating the requirement for protracted hospital stays.
7. Research is still being conducted, and this is giving patients more alternatives for treatment by examining new combinations and uses of imatinib.
Before beginning Veenat (Imatinib) capsule therapy, patients must discuss potential side effects and advantages with their doctor. To manage potential side effects and achieve the greatest results, regular medical monitoring is essential. Veenat use requires knowledge, and treatment decisions should be made in collaboration between patients and their oncologists while taking into account unique circumstances and therapeutic objectives. To manage side effects and maximise therapy efficacy, patients should be regularly watched and given supportive care.
Veenat Capsules (Imatinib) Uses:
The following conditions are treated using Veenat capsules, which contain the active component imatinib:
1. Veenat is recommended for the treatment of chronic myeloid leukaemia (CML), as well as for the treatment of CML in the accelerated phase, blast crisis, or following the failure of interferon-alpha therapy.
2. Gastrointestinal Stromal Tumours (GISTs): Veenat is indicated as adjuvant therapy following complete resection of GISTs for the treatment of unresectable and/or metastatic GISTs.
Veenat Capsules (Imatinib) Side Effects
Veenat is generally well accepted, although some people may experience some adverse effects. Typical negative consequences could be:
- Nausea and Vomiting
- Diarrhea or Constipation
- Muscle Cramps
- Fluid Retention or Edema
- Fatigue or Weakness
- Skin Rash or Itching
- Headache
- Joint Pain
- Abdominal Pain
- Low Blood Counts (Anemia, Neutropenia, Thrombocytopenia)
- Liver Function Test Abnormalities
- Elevated Blood Glucose Levels (Hyperglycemia)
- Heart Problems (e.g., Edema, Heart Failure)


Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Medicine Category
Veenat Imatinib Mesylate 400mg Tablets
Price 2500 INR / Box
Minimum Order Quantity : 100 Boxes
Dosage Form : As Per Suggestion
Origin : India
Feature : Other
Shelf Life : 2 Years
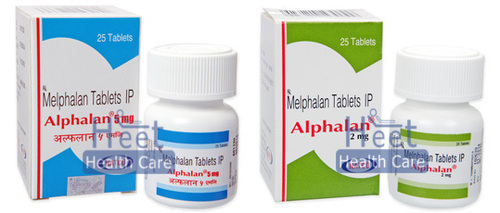
Alphalan Melphalan 2mg Melphalan 5mg Tablets
Price 5000 INR / Box
Minimum Order Quantity : 100 Boxes
Dosage Form : As Per Suggestion
Origin : India
Feature : Other
Shelf Life : 2 Years
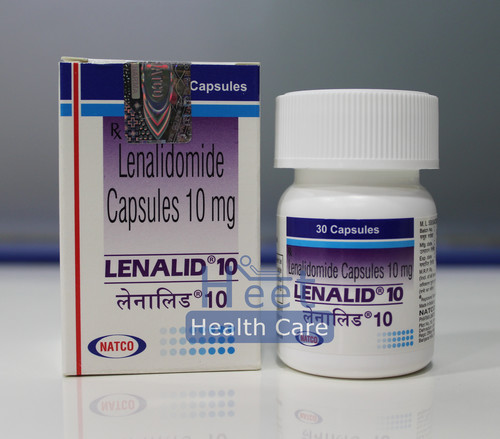
Lenalid 10 Mg Capsules
Price 4800 INR / Bottle
Minimum Order Quantity : 10 Bottles
Dosage Form : As Per Suggestion
Origin : India
Feature : Other
Shelf Life : 2 Years
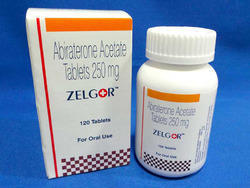
Zelgor Abiraterone Acetate 250mg Tablets
Price 18000 INR / Bottle
Minimum Order Quantity : 100 Bottles
Dosage Form : As Per Suggestion
Origin : India
Feature : Other
Shelf Life : 2 Years
 |
HEET HEALTHCARE PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |

 Send Inquiry
Send Inquiry English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese Send Inquiry
Send Inquiry